Words courtesy of Willow Durrington RM
The World Health Organization (WHO) (2011) recommends that infants are exclusively breastfed for the first six months of life and continue to receive breastmilk until two years of age. The benefits of breastfeeding are well documented for both mothers and babies and contribute significantly to improved morbidity and mortality rates through protection against infection and the development of chronic illnesses (Louis-Jacques & Stuebe, 2018; Stuebe, 2009; Victoria et al., 2016). Additionally, at a community level, breastfeeding is environmentally friendly and economical (World Alliance for Breastfeeding Action, n.d.). The Baby Friendly Health Initiative (BFHI), and its Ten Steps to Successful Breastfeeding, was initiated as a means to protect, promote, and support breastfeeding worldwide (United Nations International Children’s Emergency Fund [UNICEF] & WHO, 2018), however, the BFHI does not specifically address intrapartum factors, such as medications, which may affect breastfeeding.

So what is the impact of narcotic analgesia during labour on the breastfeeding woman and her infant? Relevant literature will be critically analysed and evaluated here, with reference to the physiology of lactation, in order to develop recommendations and strategies to enable midwives and health professionals to support the mother-infant breastfeeding relationship when narcotic analgesia has been used in labour for pain relief.
The context is parenteral narcotic analgesia, medications that are administered intramuscularly and intravenously for pain management in labour (Tiziani, 2017). Narcotic analgesia, such as opioid drugs, act by mimicking endogenous endorphins and stimulate opioid receptors in the central nervous system to reduce the receiver’s sensibility to pain, without causing loss of consciousness or inhibiting sensation (Bullock & Manias, 2016; Rankin, 2017). At present, the types of narcotic analgesia used during labour are dependent on drug availability and hospital policy, but generally include morphine, pethidine, fentanyl and, at times, their derivatives (Bullock & Manias, 2016; Government of South Australia, n.d; Queensland Clinical Guidelines, 2017; Smith et al., 2018). Side effects of narcotic drugs are reported as nausea, vomiting, drowsiness, delayed gastric emptying, reduced fetal heart rate variability, depression of the baby’s respiratory centre at birth, and potential impairment of breastfeeding (Bullock & Manias, 2016; Rankin, 2017), although these may vary depending on the drug used (Anderson, 2011; Smith et al., 2018). It is important to note that pethidine is reported as the most commonly used narcotic analgesia, however, its use is controversial in labour due to the frequency of its neonatal side effects, namely respiratory depression, sedation, and suboptimal breastfeeding behaviours (Rowe et al., 2016).
Research indicates that the use of injectable narcotics for analgesia in labour is declining due to the availability, and potential preference, of epidural analgesia (Quach et al., 2020). Despite this, an analysis of Australia’s latest perinatal data reveals that 14% (32, 361) of women who gave birth in 2018 received parenteral narcotic analgesia in labour (Australian Institute of Health and Welfare, 2020). This highlights a significant number of mother-infant dyads whose breastfeeding journey may be negatively affected.

The physiology of lactation will now be outlined to assist in understanding and recognising deviations from the normal processes. Lactation is the primary function of the breast and involves the integration of neuronal, endocrine, and autocrine processes (Bartle & Mandeno, 2019). The stages of lactation are classified as mammogenesis, lactogenesis (stages I, II, and III), and involution. Mammogenesis begins during embryonic development, heightens at puberty, and again during pregnancy to support lactation. Pregnancy changes are characterised by breast growth and the proliferation of ducts and the glandular system under the influence of oestrogen and progesterone (Bartle & Mandeno, 2019; Rankin, 2017; Wambach & Watson, 2021). Lactogenesis refers to the transition from pregnancy to lactation, through the initiation and establishment of milk secretion.
The initiation of milk synthesis commences from mid-pregnancy until day two postpartum and is classified as lactogenesis I. During this period alveolar cells differentiate into secretory cells, and prolactin stimulates these to produce milk, with colostrum able to be expressed from the breast. From day two to three postpartum, the rapid decrease in progesterone levels post birth facilitates the onset of copious milk production, known as lactogenesis II. This is anecdotally referred to as ‘milk coming in’, due to increased milk volume and maternal reports of breast fullness (Bartle & Mandeno, 2019; Rankin, 2017; Wambach & Watson, 2021). Lactogenesis III occurs from day eight to nine postpartum with milk production now under autocrine, as opposed to endocrine control. The maintenance of milk supply and establishment of breastfeeding now depends on ongoing, active milk removal from the breast. When breastfeeding frequency reduces, milk secretion decreases as a result of a build-up of inhibiting peptides. Once cessation occurs, milk secretion stops and breast tissue returns to a pre-pregnancy state, known as involution (Bartle & Mandeno, 2019; Rankin, 2017; Wambach & Watson, 2021).
It is understood that certain maternal and intrapartum factors may impair lactogenesis, in reference to intrapartum narcotic analgesia, administration is said to cause a delay in lactogenesis II, with the onset of copious milk production occurring after day three postpartum (Hildebrandt, 1999; Riordan et al., 2000; Wambach & Watson, 2021). Lind et al. (2014) found that women who received any form of labour analgesia, including parenteral narcotics, had twice the risk of delayed lactogenesis II compared to women who received no pharmacological pain relief in labour. This delay has the potential to flow on and affect the establishment of lactation, cutting off plentiful milk secretion and contributing to long term breastfeeding difficulties whereby infants, and their mothers, are deprived of the benefits of breastfeeding.

A review of the literature reveals that there is a large body of research surrounding the effectiveness, safety, and acceptability of narcotic analgesia when used for pain relief in labour, however, there is a paucity of recent data on breastfeeding outcomes. This is apparent in the recent Cochrane review by Smith et al. (2018) where the secondary objective was to examine the effect of narcotics in labour on the baby, including impact on feeding, however no clear evidence was generated, with data on breastfeeding not collected by the included studies. Despite this, seminal, retrospective, and observational studies have linked parenteral narcotic administration during labour with newborn difficulty in performing behaviours required to successfully initiate breastfeeding. This lack of high-level evidence (Love & McArthur, 2018) may be related to the perceived unethical nature of conducting a randomised controlled trial on this topic. For these reasons, caution is required when interpreting these findings due to the inability to control for external variables, such as the potential utilisation of multiple pharmacological pain relief options, differing drug types and dosages, inaccuracies in self-reported data, and other intrapartum factors, such as postpartum haemorrhage, that are also known to effect breastfeeding (Flood et al., 2019).
The success of breastfeeding is in part related to newborn behaviour. A baby is born with an innate, instinctual ability to find the breast and commence sucking, known as the breast crawl (Zanardo, 2018). Narcotic medications are highly lipid soluble, meaning they readily diffuse across the placenta and transfer into the fetus in utero, and into the newborn through breastmilk (Buckley, 2015; Rankin, 2017). Once born, infants who have been exposed to narcotic analgesia during labour are found to initially have impaired spontaneous breast seeking and feeding behaviours, namely decreased alertness, difficulty latching, and inhibited suckling when attached to the breast (Ransjo-Arvidson et al., 2001; Righard & Alade, 1990; Riordan et al., 2000; Fleet et al., 2017). Furthermore, these infants exhibit poor feeding cues, due to the sedation effects of narcotics, resulting in missed opportunities for breast stimulation (Dewey et al., 2003). The resultant poor quality and quantity, of infant suckling at the breast impacts on milk production and transfer, increases a newborns risk of developing dehydration and jaundice, and contributes to poor weight gain (Dewey et al., 2003; Riordan et al., 2000). These factors increase the likelihood of supplementation with artificial formula during the first few days postpartum, which may further impact the establishment and maintenance of lactation, if supportive breastfeeding measures are not implemented (Walker, 2015).
The extent of these neonatal impacts is attributed to the level of fetal exposure and is dependent upon the type of narcotic drug, dosing, frequency, and timing of administration (Anderson, 2011; Rankin, 2017).
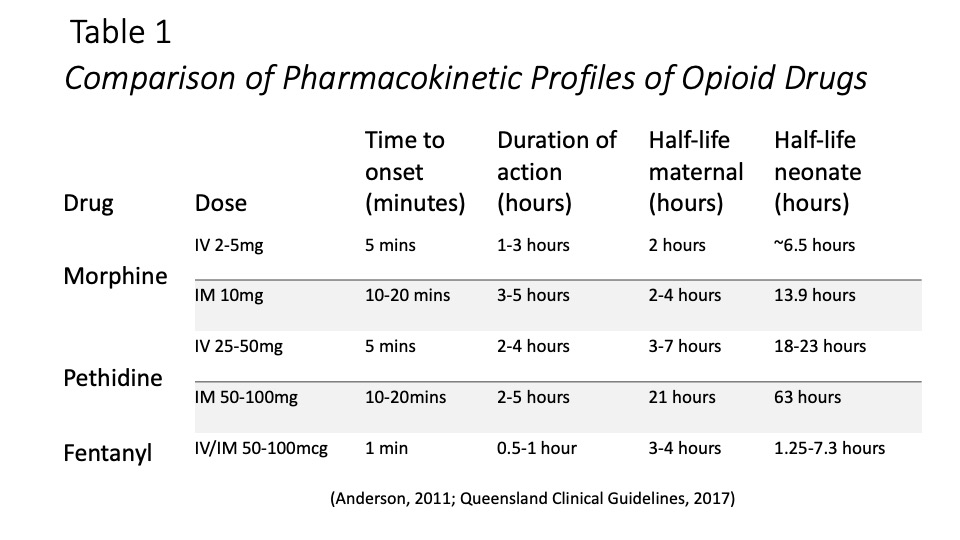
It is considered beyond the scope of this presentation to individually review all narcotic parenteral drugs used as intrapartum analgesia, however, table 1 on the slide above, highlights a comparison of the pharmacokinetic profiles of commonly used narcotics, specifically morphine, pethidine, and fentanyl (Anderson, 2011; Queensland Clinical Guidelines, 2017). It is recommended that narcotic analgesia be administered in early active labour, when birth is not expected within the next 1-2 hours, prior to 6-7cms dilatation, in order to minimise the drug’s effects on the newborn (Queensland Clinical Guidelines, 2017). Despite this, labour and delivery are notoriously unpredictable, and this can be considered an impossible prediction to make. With this in mind, the optimal narcotic analgesia for intrapartum pain relief will have a rapid onset and offset of action and fast metabolism, in order to minimise potential side effects. Both morphine and pethidine produce active metabolites, with pethidine recorded as having the longest half-life and therefore being most likely to adversely affect the neonate. Conversely, fentanyl has a quick mechanism of action, lacks an active metabolite, and is rapidly excreted, making it highly suited for intrapartum use, with fewer reported maternal and fetal side effects (Anderson, 2011; Montgomery et al., 2012). Furthermore, due to its short half-life, the amount of fentanyl present in colostrum in likely to be minimal (Anderson, 2011), particularly if administration occurred as recommended during early active labour, further reducing the potential for neonatal impacts.
It is not unsurprising that mothers whose baby has been affected by intrapartum analgesia may become discouraged with breastfeeding, potentially leading to artificial feeding, combination feeding, or unintentional early weaning (Riordan et al., 2000). Research indicates that the effects of narcotic administration are said to predominantly impact breastfeeding initiation, not duration (Riordan et al., 2000). Therefore, appropriately supported women who receive narcotic analgesia in labour should be able to go on to breastfeed successfully. Despite this current maternity care processes may present as a barrier, as discharge from hospital often occurs before effective breastfeeding is established, and timely, affordable community supports may be limited.

Recommendations to support the breastfeeding maternal-infant dyad in the context of narcotic analgesia use have been split into two streams: early interventions and management and preventative measures.
All Ten Steps to Successful Breastfeeding are considered relevant in supporting breastfeeding, however, the specific steps forming the basis for these recommendations are highlighted above.
A first-line protective measure for breastfeeding when narcotic analgesia has been given in labour is to promote immediate and uninterrupted skin-to-skin contact, between mother and baby, post birth to support early breastfeeding (UNICEF & WHO, 2018). Skin-to-skin encourages breastfeeding by supporting a newborn baby’s physiological breast seeking behaviour (Crenshaw, 2014). As previously discussed, this behaviour may be impacted by narcotic administration (Ransjo-Arvidson et al., 2001), in these instances midwives are present and are able to provide timely reassurance and practical advice to support breastfeeding. Furthermore, skin-to-skin promotes the release of maternal oxytocin which has a positive influence on bonding, milk production, and milk let down (Moore et al., 2016; Queensland Clinical Guidelines, 2016). The benefits of skin-to-skin extend beyond the immediate postpartum period and should be continued, with frequent skin-to-skin associated with exclusive breastfeeding and overcoming breastfeeding challenges (Crenshaw, 2014). Keeping mother and baby together during the initial postpartum period helps to facilitate this.
If a delay in lactogenesis II and milk coming in is diagnosed, it can be commonplace for both hospital policy and health professionals to recommend temporary formula supplementation, especially to prevent excessive weight loss, jaundice, and dehydration (Walker, 2015). However, this approach deprives mothers and babies of the optimal health outcomes of exclusive breastfeeding and has the potential to further delay lactogenesis by decreasing breast stimulation (Walker, 2015; Wambach & Watson, 2021). It is therefore recommended that midwives encourage and support these mothers to express breastmilk, which will ensure frequent stimulation and milk removal from the breast to assist in establishing lactation (Rankin, 2017). This recommendation does acknowledge that individual circumstances need to be taken into consideration and there may be situations where formula supplementation is medically appropriate (WHO, 2009). In circumstances where supplementation is required, the use of donor milk, through a milk sharing bank where screening and risk management processes occur, could be investigated as an alternative to artificial formula (Australian Breastfeeding Association, 2014).

The typical length of stay in hospital for standard maternity care is between twenty-four hours and three days (Queensland Government, 2021), for many women this means they are discharged before lactation is even established. For women who have received narcotic analgesia and are intending to breastfeed, discharge should be postponed until at least day three to account for delayed lactogenesis II and to enable appropriate intervention (Lind et al., 2014). It is recognised that this may not be a financially viable option for a health service (Bowers & Cheyne, 2016), therefore, all women should be provided with detailed information of community support services. Additionally, the creation of a breastfeeding drop-in clinic for access post-discharge will allow the provision of timely, ongoing professional and peer supports (Scott et al., 2017).
Adjustments to hospital policy and practice are also recommended. With short acting narcotics considered the most advantageous for women, newborns, and breastfeeding; policy change should occur to reflect this, with fentanyl proposed as the preferred narcotic analgesia for use in labour (Anderson, 2011; Montgomery et al., 2012). In the information available to women on narcotic analgesia, reference to breastfeeding is either lacking or literature simply states that narcotic analgesia may cause a baby to be too sleepy to breastfeed or latch correctly on the first day (Mater Mothers’ Hospital, 2017; The Royal Australian and New Zealand College of Obstetrics and Gynaecologists, 2016). Without the background knowledge on the physiology of lactation, women may not fully understand the implications of this for their breastfeeding journey, therefore, midwives need to ensure informed decision making occurs, by providing specific information regarding the potential impact of narcotic analgesia on breastfeeding, whilst remaining non-judgemental and supportive of women’s individual choices. To achieve this, midwives may require further training and professional development.
In conjunction with the previously described recommendations, strategies to decrease exposure to narcotic analgesia should also be explored, with women who avoid intrapartum narcotic analgesia more likely to exclusively breastfeed (O’Connor et al., 2018). Midwifery led models of care and birth settings should be promoted as these are recognised as the gold standard of maternity care and are associated with higher rates of non-pharmacological analgesia in labour, normal birth, and exclusive breastfeeding (Sandall et al., 2016). Furthermore, future research on the use of narcotic analgesia in labour is required and should be inclusive of neonatal impacts and breastfeeding as outcomes.
Overall, research suggests that intrapartum and postpartum factors can predict breastfeeding success, both in the short and long term, however, midwives are well placed to assist women and their infants to overcome these.

References
Anderson, D. (2011). A review of systemic opioids commonly used for labor pain relief. Journal of Midwifery & Women’s Health, 56(4), 411-418. https://doi-org.libraryproxy.griffith.edu.au/10.1111/j.1542-2011.2011.00061.x
Australian Breastfeeding Association. (2014). Position statement on donor milk. https://www.breastfeeding.asn.au/system/files/content/POL-Statement%20on%20Donor%20Milk-V3-201803.pdf
Australian Institute of Health and Welfare. (2020). Australia’s mothers and babies 2018: In brief. Perinatal statistic series no. 36. Cat. No. PER 108. Canberra: AIHW.
https://www.aihw.gov.au/getmedia/aa54e74a-bda7-4497-93ce-e0010cb66231/aihw-per-108.pdf.aspx?inline=true
Bartle, C., & Mandeno, E. (2019). Supporting the breastfeeding mother. In S. Pairman, S. K. Tracy, H. Dahlen, & L. Dixon (Eds.), Midwifery preparation for practice (4th ed., pp 671-706). Elsevier Australia.
Bowers, J., & Cheyne, H. (2016). Reducing the length of postnatal hospital stay: Implications for cost and quality of care. BMC Health Service Research, 16, 1-12. https://doi-org.libraryproxy.griffith.edu.au/10.1186/s12913-015-1214-4
Buckley, S. (2015). Hormonal physiology of childbearing: Evidence and implications for women, babies and maternity care. Childbirth Connection a program of the national partnership for women and families. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/hormonal-physiology-of-childbearing.pdf
Bullock, S., & Manias, E. (2016). Fundamentals of pharmacology ebook (8th ed.). Pearson Education Australia. https://ebookcentral-proquest-com.libraryproxy.griffith.edu.au/lib/griffith/detail.action?docID=5220653.
Crenshaw, J. T. (2014). Healthy birth practice #6: Keeping mother and baby together – It’s best for mother, baby, and breastfeeding. The Journal of Perinatal Education, 23(4), 211-217. https://www-ncbi-nlm-nih-gov.libraryproxy.griffith.edu.au/pmc/articles/PMC4235060/?tool=pmcentrez&report=abstract
Dewey, K. G., Nommsen-Rivers, L. A., Heinig, M. J., & Cohen, R. J. (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics, 112(3), 607-619. https://go-gale-com.libraryproxy.griffith.edu.au/ps/i.do?p=AONE&u=griffith&id=GALE|A108646488&v=2.1&it=r&sid=summon
Fleet, J., Jones, M., & Belan, I. (2017). The influence of intrapartum opioid use on breastfeeding experience at 6 weeks postpartum: A secondary analysis. Midwifery, 50, 106-109. https://doi.org/10.1016/j.midw.2017.03.024
Flood, M., Pollock, W., McDonald, S., Cullinane, F., & Davey, M. (2019). Primary postpartum haemorrhage, breastfeeding initiation and formula use for confinements in Victoria. Women & Birth, 32(1), s14. https://doi.org/10.1016/j.wombi.2019.07.192
Government of South Australia. (n.d.) South Australian perinatal practice guideline: Analgesia for labour and birth (Pharmacological). https://www.sahealth.sa.gov.au/wps/wcm/connect/052c4527-f3dd-4a18-809d-9d948302cdc9/Analgesia+for+Labour+and+Birth+%28Pharmacological%29_PPG_v1_1.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-052c4527-f3dd-4a18-809d-9d948302cdc9-nwU3BGG
Hildebrandt, H. M. (1999). Maternal perception of lactogenesis time: A clinical report. Journal of Human Lactation, 15(4), 317-323. https://doi-org.libraryproxy.griffith.edu.au/10.1177/089033449901500409
Lind, J. N., Perrine, C. G., & Li, R. (2014). Relationship between use of labour pain medications and delayed onset of lactation. Journal of Human Lactation, 4(30), 167-173. https://doi-org.libraryproxy.griffith.edu.au/10.1177/0890334413520189
Louis-Jacques, A., & Stuebe, A. (2018). Long-term maternal benefits to breastfeeding. Contemporary OB/GYN, 63(7), 26-29. https://web-b-ebscohost-com.libraryproxy.griffith.edu.au/ehost/pdfviewer/pdfviewer?vid=18&sid=0f832e28-5c70-4648-9248-3204fffda652%40pdc-v-sessmgr02
Love, R., & McArthur, D. B. (2018). How to read and assess for quality research. In T. L. Christenbery (Eds.), Evidence-based practice in nursing: foundations, skills, and roles (pp139-162). Springer Publishing Company.
Mater Mothers’ Hospital. (2017). Patient information: Labour and birth information. http://brochures.mater.org.au/brochures/mater-mothers-hospital/labour-and-birth-information
Montgomery, A., Hale, T. W., & The Academy of Breastfeeding Medicine. (2012). ABM clinical protocol #15: Analgesia and anesthesia for the breastfeeding mother, revised 2012. Breastfeeding Medicine, 7(6), 547-553. https://doi-org.libraryproxy.griffith.edu.au/10.1089/bfm.2012.9977
Moore, E. R., Bergman, N., Anderson, G. C., & Medley, N. (2012). Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews 2016, 11, 1-123. https://doi.org/10.1002/14651858.CD003519.pub4
O’Connor, M., Allen, J., Kelly, J., Gao, Y., Kildea, S. (2018). Predictors of breastfeeding exclusivity and duration in a hospital without baby friendly hospital initiation accreditation: A prospective cohort study. Women & Birth, 31(4), 319-324. https://doi.org/10.1016/j.wombi.2017.10.013
Quach, D., Woolley, T., Pandit, T., Rane, A., & Ray, R. A. (2020). Women’s epidural decision-making in labour: A Townsville perspective. Australian & New Zealand Journal of Obstetrics & Gynaecology, 60(6), 919-927. https://doi-org.libraryproxy.griffith.edu.au/10.1111/ajo.13199
Queensland Clinical Guidelines. (2016). Maternity and neonatal clinical guideline: Establishing breastfeeding. https://www.health.qld.gov.au/__data/assets/pdf_file/0033/139965/g-bf.pdf
Queensland Clinical Guidelines. (2017). ShortGuide: Opioids in labour. https://www.health.qld.gov.au/__data/assets/pdf_file/0035/682838/sg-opioids.pdf
Queensland Government. (2021). Your first 24 hours after having a baby. https://www.health.qld.gov.au/news-events/news/your-first-24-hours-after-having-a-baby-postpartum-postnatal
Rankin, J. (2017). Physiology in childbearing: With anatomy and related biosciences (4th ed.). Elsevier.
Ransjo-Arvidson, A., Matthiesen, A., Lilja, G., Nissen, E., Widstrom, A., & Uvnas-Moberg, K. (2001). Maternal analgesia during labour disrupts newborn behavior: Effects of breastfeeding, temperature, and crying. Birth, 28(1), 5-12. https://onlinelibrary.wiley.com/doi/epdf/10.1046/j.1523-536x.2001.00005.x?saml_referrer
Righard, L., & Alade, M. O. (1990). Effect of delivery room routines on success of first breast-feed. The Lancet, 336(8723), 1105-1107. https://doi.org/10.1016/0140-6736(90)92579-7
Riordan, J., Gross, A., Angeron, J., Krumwiede, B., & Melin, J. (2000). The effect of labour pain relief medication on neonatal suckling and breastfeeding duration. Journal of Human Lactation, 16(1), 7-12. https://doi-org.libraryproxy.griffith.edu.au/10.1177/089033440001600103
Rowe, H., Baker, T. E., & Hale, T. W. Drug therapy and breastfeeding. (2016). In K. Wambach & J. Riordan (Eds.), Breastfeeding and human lactation (5th ed., pp250-296). Jones and Bartlett Learning.
Sandall, J., Soltani, H., Gates, S., Shennan, A., & Devane, D. (2016). Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2016, 4, 1-122. http://doi.org/10.1002/14651858.CD004667.pub5
Scott, S., Pritchard, C. & Szatkowski, L. (2017). The impact of breastfeeding peer support for mothers aged under 25: A time series analysis. Maternal and Child Nutrition, 13(1), 1-9. https://doi-org.libraryproxy.griffith.edu.au/10.1111/mcn.12241
Smith, L. A., Burns, E., & Cuthbert, A. (2018). Parenteral opioids for maternal pain management in labour (review). Cochrane Database of Systematic Reviews 2018, 6. https://doi.org/10.1002/14651858.CD007396.pub3
Stuebe, A. (2009). The risks of not breastfeeding for mothers and infants. Review in Obstetrics & Gynecology, 2(4), 222-231. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2812877/pdf/RIOG002004_0222.pdf
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. (2016). Pain relief in labour and childbirth. https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Patient%20information/Pain-relief-labour-childbirth-pamphlet.pdf?ext=.pdf
Tiziani, A. (2017). Harvard’s nursing guide to drugs (10th ed.). Elsevier Australia.
United Nations International Children’s Emergency Fund., & World Health Organisation. (2018). Implementation guidance: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services – the revised Baby-friendly Hospital Initiative. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/272943/9789241513807-eng.pdf
Victoria, C. G., Bahl, R., Barros, A. J. D., Franca, G. V. A., Horton, S., Krasevec, J., Murch, S., Sankar, M. J., Walker, N., & Rollins, N. C. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms and lifelong effect. The Lancet, 387(10017), 475-490. https://doi.org/10.1016/S0140-6736(15)01024-7
Walker, M. (2015). Formula supplementation of breastfed infants: Helpful or hazardous? ICAN: Infant, Child, & Adolescent Nutrition, 7(4), 198-207. https://doi.org/10.1177/1941406415591208
Wambach, K., & Watson, G. (2021). Anatomy and physiology of lactation. In K. Wambach & B. Spencer (Eds.), Breastfeeding and human lactation (6th ed., pp48-84). Jones and Bartlett Publishing.
World Alliance for Breastfeeding Action. (n.d.). WABA activity sheet 7: Protection, support and promotion of breastfeeding. https://www.waba.org.my/resources/activitysheet/acsh7.htm
World Health Organization. (2009). Acceptable medical reasons for use of breastmilk substitutes. https://apps.who.int/iris/bitstream/handle/10665/69938/WHO_FCH_CAH_09.01_eng.pdf?sequence=1
World Health Organization. (2011). Exclusive breastfeeding for six months best for babies everywhere. https://www.who.int/news/item/15-01-2011-exclusive-breastfeeding-for-six-months-best-for-babies-everywhere
Zanardo, V. (2018). Breast crawl: the attractive warmth of the mammary areola. Acta Paediatrica, 107(10), 1673-1674. https://doi-org.libraryproxy.griffith.edu.au/10.1111/apa.14427
